STREAM
A Phase I/II, open-label study of the safety, tolerability and preliminary efficacy of hESC-derived RPE implantation in one eye in patients with single-gene retinitis pigmentosa.
Location
France : Centre Hospitalier National d’Ophtalmologie (CHNO) des Quinze-Vingts.
Resume
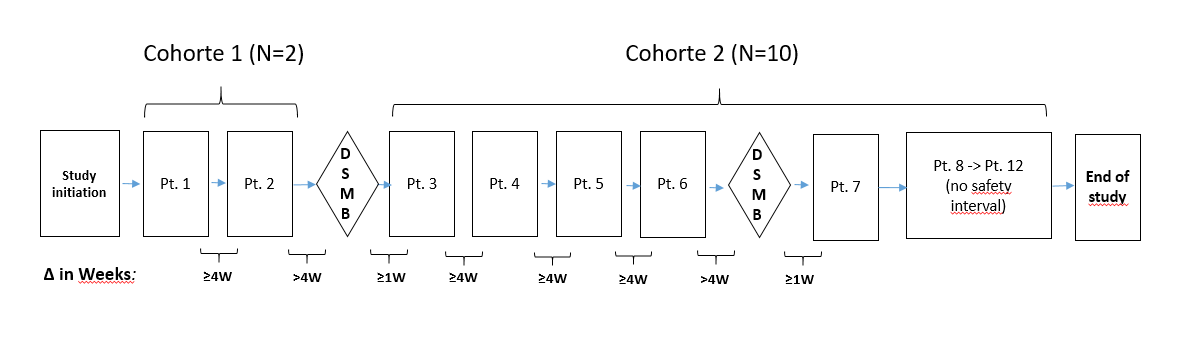
Non-randomized study, assignment of a single group of patients consisting of 2 sequential cohorts:
- First cohort of 2 patients with very advanced visual acuity loss (legally blind)
- Second cohort of 10 patients with less advanced visual acuity loss.
A total of 12 evaluable patients were recruited and divided into the two cohorts described above.
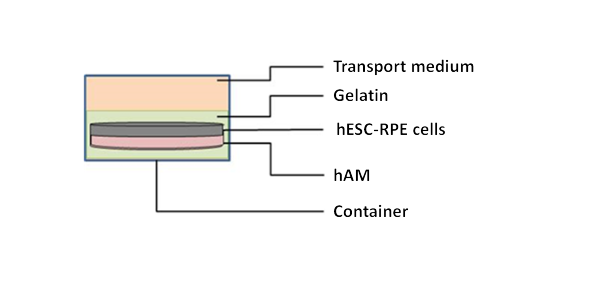
The expected follow-up for a patient is approximately 64 weeks, including 8 weeks of screening and a follow-up period of 56 weeks after implantation of the RPE (retinal epithelium) derived from hESC (human embryonic pluripotent stem cells).
After 56 weeks of follow-up, patients will be followed long-term for an additional 4 years.
The main objective is to evaluate the safety and tolerability of the implantation of the investigational medicinal product (ISTEM-01) in patients with retinitis pigmentosa.
Primary Evaluation Criteria
Safety and tolerability, as measured by the incidence of adverse events (AEs) or serious adverse events (SAEs), assessed by changes in ophthalmic examinations, laboratory parameters, vital signs, and physical examination from baseline to each visit, will be assessed for each patient over a 56-week period.
- Actual study start date: August 19, 2019
- Estimated primary completion date: August 15, 2023
- Estimated completion date: December 15, 2026
Last Update : Inclusion in the study ended in January 2023. 8 patients were included and 7 patients were treated.