AUDIOWOLF
Efficacy study of daily administration of VPA (sodium valproate) or DEPAKINE in patients with Wolfram syndrome.
Location :
2 sites :
- France: HEGP, Paris (PI: Dr Christophe Orssaud) – 11 patients already included and 5 patients treated.
- Spain : UGC Almería Periferica (PI: Dr Gema Esteban Bueno). The activation and opening of the site is planned for April 2023.
Study Summary:
European Phase II, open-label, non-randomized, single-arm study of 20 evaluable patients, 13 years of age, with sensorineural hearing loss of at least 20 dB at 8 kHz mid-high frequency (HFA), AND with documented genetic mutations in the WFS1 gene AND with at least one other documented major clinical symptom related to Wolfram syndrome (i.e., diabetes mellitus, diabetes insipidus, optic atrophy) All patients will receive three years of treatment with VPA (Depakine chrono).
NCT04940572 :
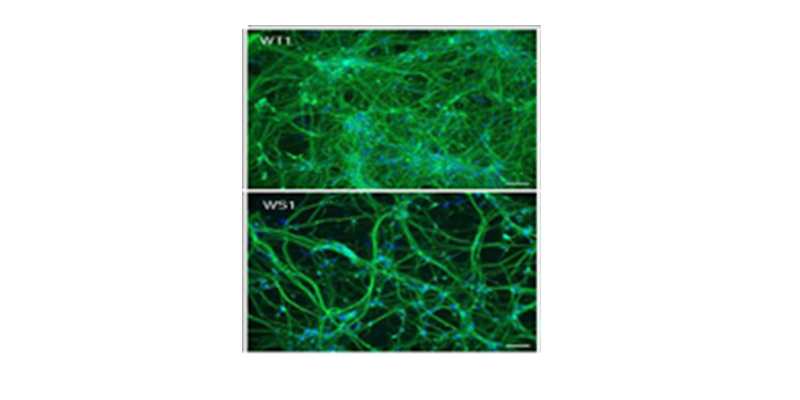
Healthy neurons (WT) and cell-derived neurons from patients with Wolfram syndrome (WS1).
The effective dose and duration of this 3-year treatment should be determined individually with the aim of achieving preservation of hearing function defined as no decrease greater than 5 dB in any one ear from baseline at 8 kHz on the high midrange frequencies and to reduce the dose of insulin and/or desmopressin required, therefore monitoring of patients’ plasma VPA concentration is necessary for dose adjustment.
In general, the goal is to achieve a plasma sodium valproate level between 40 and 100 mg/L (i.e., between 300 and 700 micromol/L).
The analysis will compare pure tone audiometry (PTA), speech interference index (SII), and high-frequency pure tone audiometry hearing test between the initial and final visits.
- Study start date: November 26, 2021
- Estimated primary completion date: November 1, 2025
- Expected completion date: December 1, 2025
Last update:
11 patients included and 5 patients treated in France, recruitment is active.
In Spain, the site will begin enrolling patients in April 2023. The clinical trial submission is being evaluated by the local competent authority.